Table of Contents
Research projects need a pilot study because it enables investigators to develop and improve their study methods before they begin their main investigation. The research procedure begins with trial performances that reveal possible obstacles and confirm investigative viability by improving the quality of outcomes. A pilot study in medical, social, and psychological research leads to more reliable and precise final research outcomes.
Research investigations usually require substantial time commitments, strong work effort, and substantial financial backing. Performing a pilot study allows researchers to reduce risks by testing procedures, maximize their resource use, and strengthen the reliability of their research outcomes. Better preparation results from this initial step mean that the final research study meets its efficiency goals while delivering important findings. This blog, ideated and written by the experts at All Assignment Help, will help students better understand various aspects associated with pilot studies.
Pilot Study – A Brief Idea
A pilot study is a small-scale trial conducted before the main research to identify design flaws, test data collection methods, and refine assessment tools. It helps researchers enhance study protocols, minimize risks, and improve execution. By evaluating practicality, resource allocation, and real-world applicability, a pilot study prevents costly mistakes that could affect final results.
As an exploratory step, it ensures the research is well-structured for optimal outcomes. However, students juggling multiple classes, exams, and assignments may struggle to dedicate enough time to it. In such cases, assignment help services can assist by handling academic writing tasks, ensuring timely submission, and helping students maintain academic balance.
To make a pilot study and following research effective, students must invest a good share of time. But when they are attending multiple classes, preparing for exams, and working on assignments simultaneously, it can be challenging to get more time to work on the pilot study. Still, those who are working on a pilot study could hire an assignment help service – such a trustworthy company can take up this load of writing students’ academic papers. They write it by complying with every instruction and help students submit it on time as well as attain good grades in it. Those who are unable to handle academic pressure can utilize such services to strike a balance in their academic as well as personal lives.
Read more: Understanding Experimental Research: Key Types and Examples
What is a Pilot Study or a Pilot Project?
A pilot study or a pilot project represents a small-scale experiment that evaluates both costs and feasibility alongside risks and time estimates as well as possible adverse events to occur before beginning research projects. Data collection methods and hypothesis validations occur before significant resource expenditure through pilot studies that researchers conduct.
When carried out successfully, a pilot study generates essential feedback concerning participant recruitment as well as data collection protocols and research duration timing. The risk of errors decreases in the main research findings because of this preliminary technique which enhances the study’s credibility. Such evaluation establishes the correctness of the research approach regarding both ethical and practical aspects particularly when human participants are included.
Purpose of a Pilot Study?
Pilot studies are conducted to calculate the practicability of some vital parts of the full-scale study. These can be broken down into four main aspects.
Below are these:
Process
The practicability of key steps in the full-scale study is monitored. For example, retention levels, recruitment rates, and eligibility criteria
Management
The problems are looked at regarding data management and also with the team included in the study. For example, were there any issues while collecting the data required for future analysis? Is the collected data highly changeable?
Resources
It includes monitoring the issues with resources and time that may arise during the main study. How long, for instance, will it take to complete the major study? Will the use of some equipment be practical?
Scientific
It deals with the monitoring of treatment safety, the estimation of treatment effect, its variety, the determination of dose levels, and its response.
Pilot studies serve as clinical trials or questionnaires.
One who is responsible for conducting a pilot study must have skills to manage all their chores timely. Be it any class, exam, or even any assignment work, or even household or professional responsibilities. If there is any lag in any work, it can affect the progress of the pilot study. If you are among those who are pursuing an online degree course, have a part-time job, and conducting a pilot study, it would be a good idea to reach out to a professional online class helper and ask them to take your online class.
This way, when someone takes care of your classes, you can use that time to conduct your pilot study successfully. Be it 12 PM or 12 AM, online class help services are available to help students pursue their higher studies successfully. Students can avail of this service at a very reasonable cost and can avail of it without thinking twice.
Objectives of a Pilot Study
Pilot studies have some specific objectives.
Here are these:
Testing Research Instruments
Data collection instruments and survey methods as well as questionnaire techniques need assessment for their measurement effectiveness. The research team needs to identify questions that have confusing wording or unclear meanings requiring revision.
Evaluating Feasibility
Researchers need to confirm whether all necessary resources, time allotments, and financial constraints permit study execution. A test needs to be performed on the participant recruitment approach to verify its success in obtaining suitable participant numbers.
Identifying Potential Issues
Research methods will assess upcoming obstacles in the process of gathering data, processing the information, and maintaining participant connection. The main study will encounter logistical problems that require understanding before its execution.
Enhancing Data Collection Methods
The researcher works to improve both quantitative and qualitative data acquisition procedures. Different testing methods were used to evaluate both data collection accuracy levels and operational efficiency. This step is very crucial in any pilot study and students must pay close attention while conducting it.
Assessing Data Analysis Procedures
Several suitable quantitative statistical tests and qualitative analysis methods need assessment. The study’s outcome measures need to match the research objectives for proper assessment.
Students from the Biology or psychology stream frequently face issues when executing this step. It is highly recommended that they hire a biology assignment help service or connect with an assignment maker asking them can you do my psychology assignment for me? Students can expect quick support from expert assignment writers and help them complete their assignments so that they can focus better on completing this key step in their pilot study.
Read more: How to Write a Research Journal?
Steps to Conduct a Pilot Study
There are set rules to conduct a pilot study and students must adhere to these.
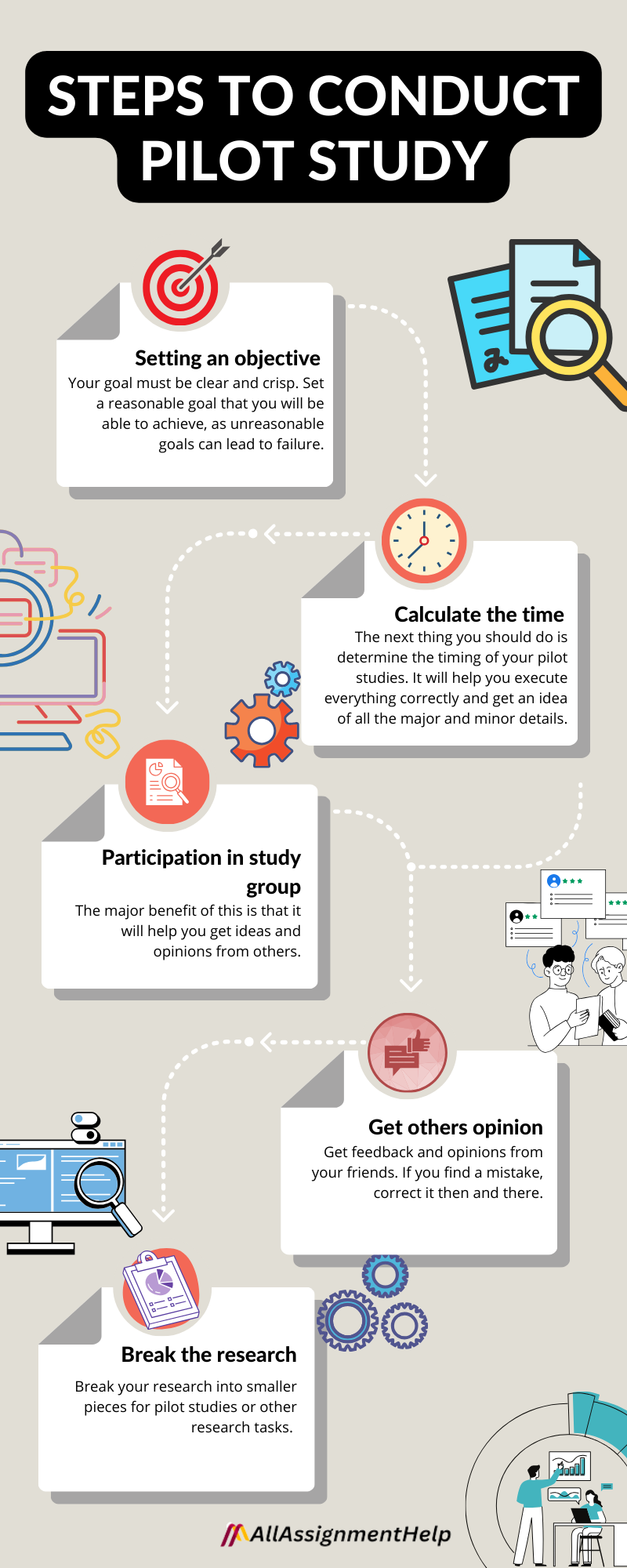
Listed below are the steps for assisting you in writing an effective pilot study for your research:
Set an objective
The goal of your pilot study must be clear. Without a vision, discussing and proceeding with the other steps of your research studies would be a waste of time. Your goal must be clear and crisp. Set a reasonable goal that you will be able to achieve, as unreasonable goals can lead to failure.
Calculate the time
Once you have set a goal and made up your mind that you will hit it, The next thing you should do is determine the timing of your pilot studies. It will help you execute everything correctly and get an idea of all the major and minor details.
Be a part of study groups
Actively be a part of different study groups. The major benefit of this is that it will help you get ideas and opinions from others.
Get third parties’ opinions
Get feedback and opinions from your friends. If you find a mistake, correct it then and there.
Breaking the research
Break your research into smaller pieces for pilot studies or other research tasks.
By following the above-mentioned steps, one can craft a proper pilot study. If you are creating a pilot study for your clinical research, then you need to be extra careful to have a successful outcome.
Do you need to submit a pilot study but are unsure of its nitty-gritty? If your answer is yes, do not feel guilty at all. Many students face this confusion. Only when you start concentrating on your studies by taking all other pressure out of your mind, you will successfully be able to address all your confusion. However, this is ideally not possible for someone who is pursuing a bachelor’s, master’s, or a PhD course. But, when students are planning to get assistance from a third party by searching I want to pay for my assignment online, they can find many companies available to take care of their papers. Signing up with one from the top of the search list and giving them the responsibility of completing assignments can be a good way to get time to invest in pilot studies.
Benefits of Conducting a Pilot Study
The implementation of a pilot study comes with many advantages which result in improved main study quality.
Some of these advantages include:
1. Identifying and Resolving Issues
- Research design errors become detectable through pilot studies which guide necessary alterations before starting the complete investigation.
- Researchers gain the opportunity to improve accuracy in their experiments by modifying their data collection systems and their research methods.
2. Assessing Feasibility
- Research feasibility is established through the assessment of the available resources to see if the project will finish on schedule.
- The study review system helps collect data regarding the effectiveness and sustainability of recruitment methods.
3. Improving Data Collection Methods
- The team working on the project should upgrade all survey questionnaires along with interview questions and data recording methods to achieve maximum precision and reliability.
- The testing process enables researchers to locate the most suitable data collection method.
4. Minimizing Risks
- Researchers identify risks before prevention measures allow for a smooth execution process of their final study.
- Research professionals need to evaluate ethical elements when working in specific sensitive research fields.
5. Enhancing Research Validity
- A pilot study enables main research to achieve valid reliable outcomes that decrease potential bias and results error.
- The research process benefits from pilot studies because they enable scientists to analyze and enhance their main questions as well as test hypotheses for the principal research.
Considering the benefits, students must take up a pilot study/project with the aim of making their main paper (research paper or PhD thesis) flawless.
Online exams often pose blockades to the students conducting and concentrating on a pilot study. The eerie feeling of unpreparedness for the exam can harm both exam performance as well as their pilot study. Realizing this issue, many students look for an online exam helper wondering if can I pay someone to do my online exam. Genuine online exam helpers comprise experts having years of experience in handling popular LMS (like Moodle, Edmodo, blackboard, etc.) used by colleges across the globe. They can sign in and appear in online exams without any issues and help students gain high grades.
Read more: Case Studies: Grasp a Complete Knowledge of It
Conclusion
Experimental research depends on pilot studies because they help researchers achieve a well-deliberated final study with enhanced accuracy and effectiveness. Research preliminary tests enable scientists to detect possible difficulties which they can use for methodology refinement to increase study validity. A research plan becomes more reliable and of better quality when it incorporates pilot study methods through traditional or remote approaches.
The implementation of a properly ordered pilot study enables researchers to strengthen their approach while acquiring useful understanding which benefits their final research implementation. The analysis conducted in the pilot study contributes to the production of comprehensive impactful research findings.
Frequently Asked Questions
| Question 1: Is a pilot study essential for research projects? Answer: There is no requirement that one must include a pilot study in their research. But it is still recommended that the researchers understand its importance and include it in their work. The main benefit is that it will prevent you from eliminating all errors and carrying out successful research. |
| Question 2: What are the two important aspects of a pilot study in research? Answer: There are two major benefits to a pilot study. One is that it will help you justify the main research questions. The second one is that it also improves the feasibility of your research study. |
